Background
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative treatment option frequently used in patients with acute lymphoblastic leukemia (ALL). However, relapse after allo-HCT remains the main concern because it is associated with poor outcomes, with a long-term survival rate < 10 %. There is no standard treatment for patients with ALL who relapsed after allo-HCT.
Second allo-HCT (allo-HCT2) is a curative treatment option for patients with ALL who relapsed after the first allo-HCT (allo-HCT1). However, data on allo-HCT2 in patients with ALL are limited, and the indications and outcomes of allo-HCT2 are unclear.
Therefore, this study aimed to investigate the outcomes and prognostic factors of patients who underwent allo-HCT2 after ALL relapse.
Methods
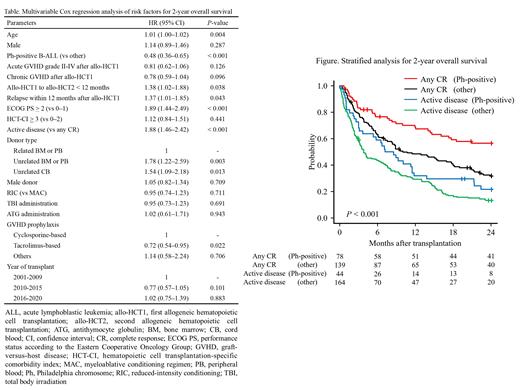
This study included 425 adult patients with ALL who underwent allo-HCT2 after relapse between January 2001 and December 2020. The primary endpoint was 2-year overall survival (OS), which was evaluated using the log-rank test. Prognostic factors for the primary endpoint were evaluated using the multivariable Cox proportional hazards model. The variables considered in the Cox proportional hazards model are listed in the Table. The impacts of the candidate factors are shown as hazard ratios (HRs) and 95% confidence intervals (CIs). Additionally, we performed an analysis stratified by disease and disease status because Philadelphia chromosome (Ph)-positive B-ALL could affect the treatment and disease status at allo-HCT2 and the outcome.
Results
The median age at allo-HCT2 was 36 years (interquartile range; 27-48 years) and 220 patients (52%) were male. According to the Eastern Cooperative Oncology Group, 94 patients (22%) had a performance status of > 2. There were 235 patients (55%) with Ph-negative B-ALL, 122 patients (29%) with Ph-positive B-ALL, and 68 patients (16%) with T-ALL. Regarding disease status at allo-HCT2, 217 patients (51%) had any complete response (CR). One hundred forty-eight patients (35%) received myeloablative conditioning regimen. Ninety patients (21%) received related bone marrow (BM) or peripheral blood (PB) transplantation, 137 patients (32%) received unrelated BM or PB transplantation, and 198 patients (47%) received unrelated cord blood (CB) transplantation.
The median observation time of survivor after allo-HCT2 was 22.3 (interquartile range; 6.3-82.5) months and 2-year OS was 28.0% (95% CI, 23.7-32.4). After stratified disease and disease status, the 2-year OS was 56.5% (95% CI, 44.6-66.8), 31.7% (95% CI, 24.0-39.7), 21.5% (95% CI, 10.6-34.9), and 13.1% (95% CI, 8.4-18.8) in any CR (Ph-positive B-ALL), any CR (other), active disease (Ph-positive B-ALL), and active disease (other), respectively ( P < 0.001) (Figure).
In multivariable analysis, Ph-positive B-ALL (HR, 0.48; 95% CI, 0.36-0.65; P < 0.001), tacrolimus-based GVHD prophylaxis at allo-HCT2 (HR, 0.72; 95% CI, 0.54-0.95; P = 0.022) were identified as significantly positive prognostic factors for 2-year OS, while allo-HCT1 to allo-HCT2 < 12 months (HR, 1.38; 95% CI, 1.02-1.88; P = 0.038), relapse within 12 months after allo-HCT1 (HR, 1.37; 95% CI, 1.01-1.85; P = 0.043), ECOG PS > 2 (HR, 1.89; 95% CI, 1.44-2.49; P < 0.001), active disease at allo-HCT2 (HR, 1.88; 95% CI, 1.46-2.42; P < 0.001), unrelated BM or PB transplantation at allo-HCT2 (HR, 1.78; 95% CI, 1.22-2.59; P = 0.003), and CB transplantation at allo-HCT2 (HR, 1.54; 95% CI, 1.09-2.18; P = 0.013) were identified as significantly negative prognostic factors for 2-year OS.
Conclusion
In this study, we demonstrate that the 2-year OS was 28.0% in patients with ALL who underwent allo-HCT2 and identified the prognostic factors that may guide patients to benefit from allo-HCT2.
Disclosures
Najima:Nippon Shinyaku Co., Ltd.: Speakers Bureau; Sumitomo Pharma Co., Ltd.: Speakers Bureau; Takeda Pharmaceutical Company Limited.: Speakers Bureau; Daiichi Sankyo Co. Ltd.: Consultancy, Speakers Bureau; AbbVie GK: Speakers Bureau; Amgen Inc.: Speakers Bureau; Bristol-Myers Squibb K.K.: Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Speakers Bureau; CSL Behring K.K.: Speakers Bureau; Janssen Pharmaceutical K.K.: Speakers Bureau; Novartis Pharma K.K.: Speakers Bureau; Kyowa Kirin Co., Ltd.: Speakers Bureau; Otsuka Pharmaceutical Co., Ltd.: Speakers Bureau; Astellas Pharma Inc.: Consultancy, Speakers Bureau. Tanaka:Sumitomo Pharma: Speakers Bureau; Otsuka Pharmaceutical: Speakers Bureau; MSD: Speakers Bureau; Kyowa-Kirin: Speakers Bureau; Daiichi Sankyo: Speakers Bureau; Chugai Pharmaceutical: Speakers Bureau; Astellas Phrama: Speakers Bureau; Asahi Kasei Pharma: Speakers Bureau; Abbvie: Speakers Bureau; Pfizer: Speakers Bureau. Doki:Novartis Pharma K.K.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria. Ota:Janssen: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Novartis: Speakers Bureau; Amgen: Speakers Bureau; AstraZeneca: Speakers Bureau. Kanda:Towa Pharma: Speakers Bureau; AbbVie: Research Funding, Speakers Bureau; CSL Behring: Speakers Bureau; Japan Blood Products Organization: Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Research Funding, Speakers Bureau; AstraZeneca: Speakers Bureau; Human Life CORD: Speakers Bureau; Sumitomo Pharma: Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Takeda Pharmaceutical: Research Funding, Speakers Bureau; Meiji Seika Pharma: Speakers Bureau; Asahi Kasei Pharma: Research Funding, Speakers Bureau; Daiichi Sankyo: Research Funding, Speakers Bureau; Saitama Hokeni Kyokai: Speakers Bureau; MSD: Speakers Bureau; Kyowa Kirin: Research Funding, Speakers Bureau; Janssen Pharmaceutical: Speakers Bureau; Sanofi: Speakers Bureau; Pfizer: Speakers Bureau; Chugai Pharmaceutical: Research Funding, Speakers Bureau; Novartis: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Eisai: Research Funding, Speakers Bureau; Shionogi Pharma: Research Funding; Precision: Speakers Bureau; Alexion Pharma: Speakers Bureau; FUJIFILM Wako Pure Chemical: Speakers Bureau; Wakunaga Pharmaceutical: Speakers Bureau; Taiho Pharmaceutical: Research Funding; Nippon Kayaku: Research Funding; JCR Pharmaceuticals: Research Funding. Atsuta:Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; JCR Pharmaceuticals Co., Ltd.: Consultancy; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau; Novartis Pharma KK: Speakers Bureau; Meiji Seika Pharma Co, Ltd.: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal